Unanswered Questions as FDA Allows Pfizer Vaccine Rollout
Updated
By Jefferey Jaxen
Just in time for the holiday rush, America’s Covid vaccine is here! Experts and officials who claim to represent the highest ideals science has to offer are now on individual and collective public relations tours to sell the product to a largely dubious public.
On Thursday the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) met to discuss the limited trial data submitted to them from Pfizer’ Covid vaccine candidate.
After hours of discussion, the committee gave its blessing, in a 13-4 vote. Twenty-four hours later, the FDA granted the company’s experimental shot an Emergency Use Authorization (EUA) with “the first inoculations expected within days” wrote Reuters.
Early media narratives, pushed by health officials, promised an end to lockdowns once the vaccine was here. But once it looked like a vaccine may actually come to fruition, it was a different story. Now, these same media outlets and health officials have changed course, saying lockdowns and masks will still be needed even after the vaccine arrives.
As the goalposts continue to move, people have grown distrustful of the empty promises.
Nevertheless, the gears have been activated and are beginning to churn as America’s great experiential vaccine distribution goes live. Frontline workers—doctors, nurses, firefighters, emergency medical technicians and others—have all expressed their hesitancy at being part of the expanded experiential trials for Pfizer’s shots. There is a lack of clear evidence the benefits will outweigh the risk at this stage.
Meanwhile, America’s elderly and those in long term care facilities aren’t allowed the luxury to be hesitant or question the shot. They are first in line in the official Phase 1a vaccine distribution plan.
The FDA’s decision was based on Pfizer trial data that ended on November 14th. The vaccinated group in that data set reported two deaths, four cases of Bell’s Palsy and eight cases of appendicitis. These were not considered red flags.
Additionally, at the time of the VRBPAC/FDA green light, nearly a month of data was unaccounted for. Were there more deaths? Serious adverse events? Further data that would lower the shot’s inflated efficacy?
No one knows. We do know that in the UK, which rolled out Pfizer’s experimental shot one week earlier, two heath workers had allergic reactions after receiving the jab.
Applauding the UK’s real time transparency of its recent allergic reactions, USA Patient Network Board of Directors and founder of Woody Matters Kim Witczac told VRBPAC there needs to be the same transparent, real time process for catching and communicating safety signals to the public.
“Trials should be directly testing the endpoints that matter” stated Peter Doshi, PhD in his VRBPAC testimony. Doshi previously, in his October BMJ article, neutralized the media hype being erroneously echoed by pundits like Paul Offit and even Moderna itself, that the vaccines in trial were testing to stop moderate and severe illness; such as the ability to reduce deaths, ICU use and hospitalization. Turns out their trial designs were only looking at primary endpoints of a mild symptom and a lab-positive PCR test.
Pfizer’s study protocol says that in the seven days after vaccination, do not test, unless in the investigator’s opinion, clinical symptoms suggest Covid rather than vaccine side effects.
Doshi pointed out to the VRBPAC committee that Pfizer’s trial investigators were put in a position to make guesses as to what group trial participants were in, due not only to the similarity, but also the frequency, of several vaccine adverse events and mild Covid symptoms. It is still not clear if these guesses were included in the investigators’ judgement when determining the trial’s primary endpoint.
What is clear from Pfizer’s data used by the FDA to make its EUA was that its experimental shot lacked data and evidence on its effectiveness against:
• Mortality
• Transmission of Covid-19 from individuals who are infected despite vaccination
• Individuals previous infected with Covid-19.
There is also no data on the shot’s effect on pregnant women, or on fertility generally. It remains to be seen if vaccinators will inform patients that this shot was authorized under an EUA. Will patients understand what that means? Is there a risk the fact sheets communicating this point will fall short depriving the current Phase 1a general public trial participants of true informed consent?
Several comments during the VRBPAC deliberations urged that Pfizer’s data be made publicly available for independent verification – disclosures that pharmaceutical corporations typically shy away from.
The FDA’s EUA requirement is for an intervention to meet at least a 50% efficacy rate. Given the trial data relied upon, it is still unknown if Pfizer’s experimental vaccine can generate protection which extends to recipients six months or even a year out.
Another point of contention is that Pfizer plans to offer its experimental shot to all placebo recipients in their trail “after completing 6 months of follow-up after Dose 2” and that’s if they haven’t already requested and received it before that.
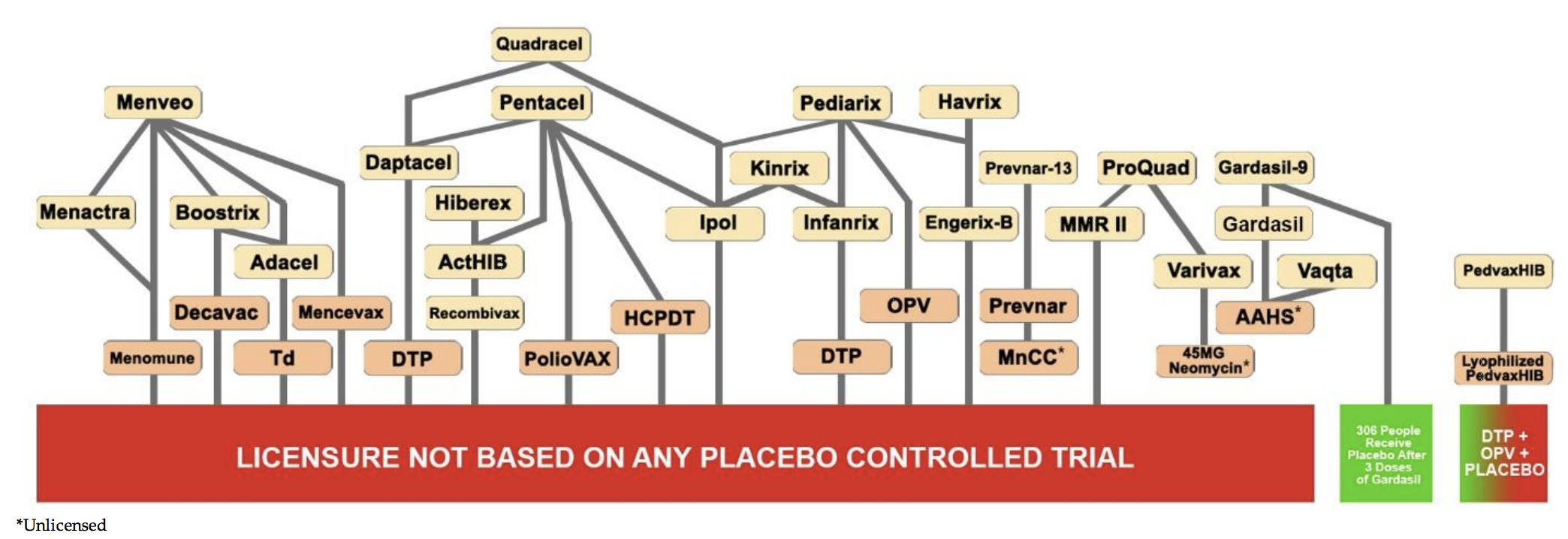
Although there was discussion around this point during the VRBPAC committee, it appeared the consensus was that it would be unethical to deny trial participants the shot. A convenient excuse that has previously allowed vaccine-makers to exclude placebos from ‘gold standard’ trials in nearly all shots recommended in America. Will the eventual pre-licensure trails used for full authorization of a Covid vaccine also be green-lighted without a true inert placebo group?
Steven Goodman, MD, MHS, PhD suggested a ‘crossover design’ in which, after 6 months, the placebo participants still receive the shot while the initial vaccine group reviews a placebo.
Yet such a design still forever eliminates any long term safety monitoring of harms from an purely unvaccinated placebo group. Goodman made the admission that “at some point in the evolution of vaccine testing, we may no longer be able to conduct placebo controlled trials of any type in settings where vaccines are available.”
At the end of the day, VRBPAC didn’t have to authorize the vaccine and the FDA didn’t have to grant it an EUA…but they did. In light of the litany of unanswered questions that result from limited trial data, an expanded access program could have been set up for those who wanted them. Despite what the media has made us believe, access to these experimental shots didn’t require authorization. The voices who wished to see clear evidence that the benefits of these vaccines outweighed the risk didn’t win out this time.
Speaking to Business Insider, Professor A. Oveta Fuller, a microbiology and African studies professor at the University of Michigan, said:
”Two more months of an expanded access Phase 3 clinical study…would bring evidence-based results that would inform researchers, broaden diversity of enrollment and build public trust in taking (or not, for some) a safe, better tested efficacious vaccine,”
Now it is the duty of the public and sensible media outlets to boldly face the headwinds of Big Pharma PR departments and captured agencies and report the truths as they find them surrounding Pfizer’s experimental shot.