WHY DO VACCINE SAFETY STUDIES CUT CORNERS?
Updated
By Jefferey Jaxen
The authorities will tell you vaccines have undergone the ‘gold standard’ in safety testing.
They will insist immunizations have a long safety profile.
They will claim that the chance of harm from any of Big Pharma’s injectable product lines is one-in-a-million.
But are they correct?
Almost surely not. But don’t take our word for it.
The BMJ medical journal has never been one to shy away from delivering medical findings, nor to demand transparency while other journals play it safe. The BMJ’s recent analysis looks at the World Health Organization’s (WHO) rollout of the malaria vaccine in Africa.
Professors Peter Aaby and Christine Stabell Benn, two authors of the recent BMJ analysis, are asking seemingly obvious questions about vaccine safety that much of the medical community works hard to ignore.
The analysis asks “can safety questions be answered after only 24 months?” Drug trials often allow what are called surrogate markers as evidence of a drug’s efficacy in clinical trials.
For example, controversial statin drugs and cholesterol levels for heart disease. Even the FDA has allowed accelerated approval of drugs for serious diseases based on surrogates deemed “reasonably likely” to predict a real outcome.
Because of the urgency of improving malaria control in Africa, the WHO intends to decide on extending the vaccine to other African countries after only 24 months. They’re using the prevention of “severe malaria” as the reason, a stand-in for overall mortality.
But as The BMJ analysis authors explain:
“Severe malaria is not a good marker for all cause mortality; it is not even a good marker for malaria mortality, as data indicate that case fatality from severe malaria might be higher in the malaria vaccine group.”
In 1978, Professor Aaby established the Bandim Health Project (BHP). The BHP was a health and demographic surveillance system site in Guinea-Bissau in West Africa.
At the time, child mortality was very high in Guinea-Bissau. Malnutrition was assumed to be the main cause. A study was initiated to determine why children were malnourished. There was no community vaccination program in Guinea-Bissau until BHP initiated one in 1981 under Aaby’s direction. Later in 1986, a full-fledged national program was implemented, with UNICEF support continuing today.
According to Aaby, he and his research team did “one of the most spectacular things [they] have done” in 1989, as the WHO recommended a new measles vaccine for use in Guinea-Bissau and Senegal vaccination campaigns.
Aaby and his team showed the new vaccine was associated with two times higher female mortality. Similar findings were seen in Haiti and Sudan by other researchers.
After initially discounting Aaby’s findings, the WHO eventually withdrew the new measles vaccine “with no real explanation” and making “no attempt to understand what has happened.” As Aaby explains:
“[A] vaccine which is fully protective against a specific disease but associated with highly mortality…that’s nowhere in the textbooks.”
Currently in Africa, Phase III trials of the RTS,S/AS01 malaria vaccine identified three safety concerns: higher risks of meningitis, cerebral malaria, and doubled female mortality. The BMJ analysis states:
“An early decision after 24 months might be biased in favour of the vaccine, which was more efficacious in the first year of follow-up in the phase III trials; the relative risks of both cerebral malaria and female mortality increased after the booster dose at 20 months”
Aaby made big waves throughout the scientific and medical communities with his 2018 study titled Evidence of Increase in Mortality After the Introduction of Diphtheria–Tetanus–Pertussis Vaccine to Children Aged 6–35 Months in Guinea-Bissau: A Time for Reflection? Aaby and his team concluded:
“…6–35 months old DTP-vaccinated children tended to have higher mortality than DTP-unvaccinated children. All studies of the introduction of DTP have found increased overall mortality.”
The BMJ authors suggest the WHO take a more common sense and scientific approach by recommending the pilot studies use “overall mortality” to assess vaccine performance rather than an artificial surrogate placeholder. In addition, they recommend populations are followed and studied for the full 4-5 years before a decision on rollout is made.
WHO’s safety monitoring problem isn’t just an isolated incident in a far-away continent.
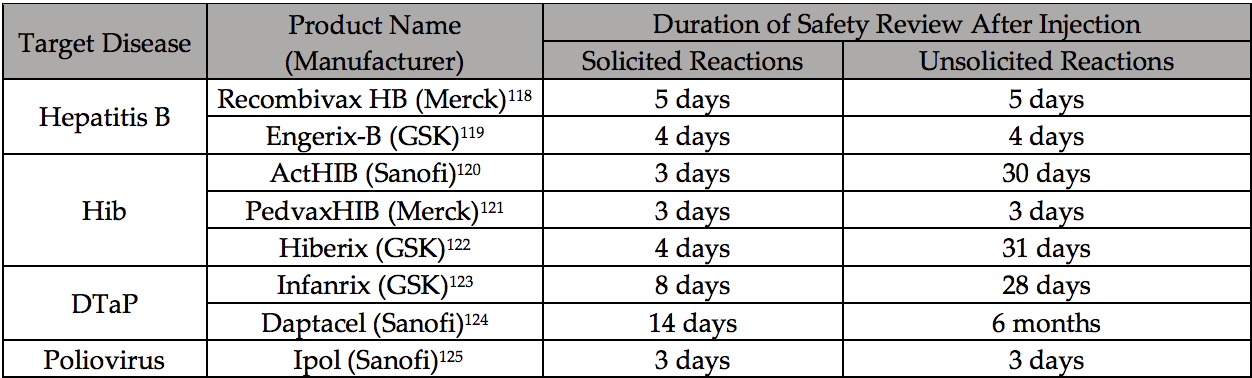
In 2017 The Informed Consent Action Network (ICAN) served a legal notice to the The U.S. Department of Health and Human Services (HHS), seeking answers to a list of basic questions regarding their Congressionally-mandated duties of vaccine safety oversight in the U.S.
U.S. regulatory and health agencies claim that vaccines undergo ‘gold standard’ testing and robust safety monitoring. But ICAN’s court win revealed the HHS documentation it relies upon to license the vaccines— recommended to babies at two months of life—was too short to capture any resulting chronic health conditions.
After scientists began to access the VSD to conduct studies that revealed vaccine harm, in 2001 HHS moved the VSD to a health industry trade association. This made the VSD data no longer subject to FOIA, and assured that only the scientists and studies HHS approves are able to use the VSD. Other abuses and unethical behavior surrounding suppression of long-term vaccine safety studies using the VSD data are detailed in ICAN’s reply to HHS. It can be accessed here.
Everyone who receives a vaccine is considered post-marketing surveillance. Slick science has forced the citizenry, without their knowledge, to take part in the final phase of medical testing for vaccine safety.
Were you given informed consent that you are a research subject, before accepting your vaccination? Did you know about and understand the serious adverse events your vaccine was known to cause before injection?