Gates-Funded Needle-Free Drugs & Vaccines are on the Horizon
Updated
Bill Gates is at it again. With funding from the U.S. Centers for Disease Control and Prevention (CDC) and the Bill and Melinda Gates Foundation, the first-ever clinical trial of a microarray injection-free vaccine has concluded, with children in Africa as young as nine months old “painlessly” being given measles and rubella vaccines through what looks like a small round peel and stick band-aid. The microarray patches are being touted as the future of vaccination in low-income and pandemic settings. Indeed, the highly profitable microneedle market is projected to reach $2.3 billion by 2030 for the flu vaccine alone.
The Gates-funded study was conducted by Micron Biomedical, a life science company based in Atlanta, and presented last week at the MICRONEEDLES 2023 conference in Seattle, Washington. The study evaluated the safety, immunogenicity, and acceptability of the leading commercially available measles-rubella (MR) vaccine from the Serum Institute of India using Micron’s microarray technology. In the trial, 45 adults, 120 toddlers (15-18 months old), and 120 infants (9-10 months old) were enrolled in an age de-escalation manner and randomized to receive the MR vaccine either by Micron’s microarray or by subcutaneous (SC) injection.
While study results of the Phase 1/2 trial are not yet published, Micron reported in a May 17, 2023 press release that vaccination by microarray was safe and well tolerated with no allergic reactions or related serious adverse events. Day-42 immunogenicity showed high and similar seroprotection rates for measles and rubella in all cohorts for both the microarray (93.2% – 100%) and SC injection (89.8% – 100%) groups. Likewise, in infants who were MR-vaccine naïve at the start of the trial, seroconversion rates were high and similar for both the microarray (92.9% -100%) and SC injection groups (89.7%-100%). Over 90% of the parents of toddlers and infants enrolled in the trial who took part in an acceptability survey said that the microarray technology would be better than SC injection to give vaccines to children.
The trial results are good news for Gates, Micron, and the dozens of other companies testing microarray technology, as the technology aims to “significantly simplify the transport, storage, and administration of vaccines that are traditionally delivered via injection and eliminate sharp waste.” For example, besides the MR trial, preclinical development is underway for human papillomavirus (HPV), typhoid, and rotavirus, while trials for vaccine patches to combat seasonal influenza, hepatitis B, and COVID-19 are ongoing. Despite the massive failure of the mRNA COVID-19 “vaccine” campaign, James Goodson, co-investigator for the study and Senior Scientist and Epidemiologist in the Global Immunization Division at the CDC, remarked:
“Supporting innovations in vaccine delivery is critical to addressing ongoing health inequities. This clinical trial is an important step forward in the critical development pathway for the MR microarray patch toward licensure and a major contribution that may help shape future approaches to reaching children and families with life-saving vaccines around the world.”
In 2017, Micron, which currently has $17 million in Series A funding for its microneedle project, received a $2.2 million grant from the Gates Foundation to develop the MR patch. Gates had previously awarded Micron $2.5 million to develop a microneedle patch for polio as part of the foundation’s endeavor to eradicate polio, a quest he has relentlessly pursued for decades. The company received another $900K from Gates in 2022, and in the last several months, Micron has received substantial funding from investors LTS Lohmann and Global Health Investment Corporation.
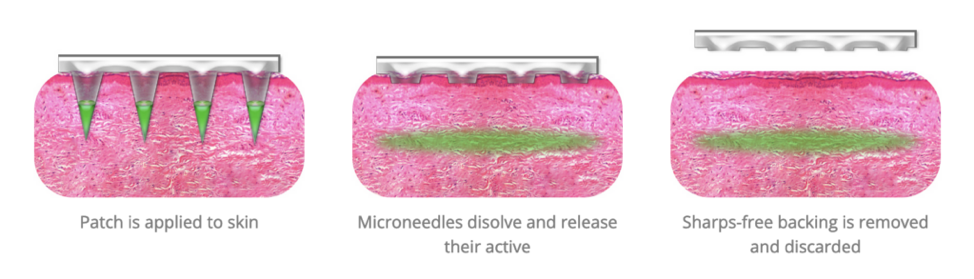
Micron’s “peel & stick” microneedle patch is applied by pressing it against the skin like a Band-Aid, allowing for a minimally invasive approach to inject vaccines that “eliminates pain and fear associated with injections and allows for self-administration with little to no training.” Meaning literally anyone can administer it. Micron’s website explains that once applied to the skin, the microneedles penetrate the upper layers of the skin and dissolve rapidly, releasing their drugs or vaccines. The patch is removed shortly after, and the microneedles dissolve into the skin.
Screenshot / Micron Biomedical
According to Micron, this method improves efficacy as it targets dermal immune system cells, which have been shown to stimulate enhanced immune responses when delivered by microneedle. Also, Micron explains that the microneedle patch targets blood capillaries just below the epidermis, amplifying the pharmacokinetic and pharmacodynamic effects of certain drugs. Likewise, Micron’s proprietary formulation and manufacturing process allows for the reduction and sometimes elimination of the requirement to keep vaccines cold, which has been an obstacle to the current mRNA COVID-19 injections. Lastly, Micron exclaims that the “versatile” microneedle patches are “suitable for a wide range of active (therapeutics and vaccines) and inactive compounds including small molecule, nucleic acids, peptides, proteins, and (the dangerous) micro/nano-particles.”
Co-founded by Mark Prausnitz, Ph.D., and Steven Damon, Micron follows in the footsteps of the world’s first transdermal patch marketed in 1979 for motion sickness. Other patches followed, but because the skin is such an effective barrier, only certain kinds of molecules can be delivered this way. Thus, scientists have spent decades searching for alternatives. In 1995, following his idea of a microneedle, Prausnitz began working at the Georgia Institute of Technology, taking advantage of its microfabrication experts. According to Gates-funded Gavi, despite having no experience with pharmaceuticals and drug delivery, the experts drew on techniques they developed in the computer chip industry to manufacture projections that were tiny enough to execute the goal of delivering vaccines into the skin. After that, the next challenge is incorporating active ingredients into the microneedle patches.
There is no doubt microneedle delivery is the horizon to create thermostable mRNA COVID-19 jabs. Similarly, besides for COVID, essentially every disease platform aiming to take advantage of the long-awaited introduction of mRNA technology thanks to the pandemic may examine the vast future of vaccination that exists with microneedles. And they may even be printed using a standalone microneedle patch (MNP) vaccine printer to be administered by anyone. Yet, like with the COVID vaccine trials, there has been no mention thus far about the long-term safety of the drug delivery system after it enters the skin and travels through the body. Consequently, serious safety considerations appear inevitable.