How GSK’s RSV Vaccine Safety Signal Opens Up Investigation for All Maternal Vaccines
Updated
At the ReSViNET Conference in Lisbon, Portugal, in February 2023, GSK Vice President of Vaccine Development Ilse Dieussaert discloses why GSK abandoned its maternal RSVPreF3 vaccine trial last February. The study was a phase 3, double-blinded, saline placebo-controlled trial with participants across 24 different high and low-middle-income countries. The pregnant women were followed up to six months post birth, and the infants up to a year. Dieussaert says a safety signal arose from “imbalances in the proportions of preterm births and neonatal deaths in the RSVPreF3 Mat group versus the placebo group.”
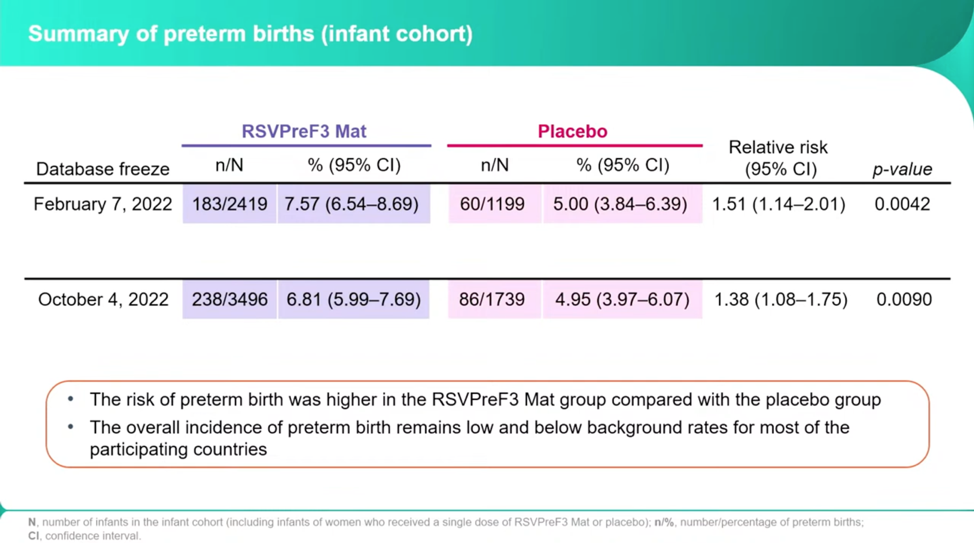
The researchers froze the trial in part because they found a larger number of preterm births in the RSVPreF3 than the placebo, 7.57 percent of the vaccine births and 5 percent of the placebo births, or a 1.51 relative risk. Dieussaert stresses that after all the remaining births were reported in October, these numbers decreased to 6.81 percent and 4.95 percent, a relative risk of 1.38.
Twice as Many Neonatal Deaths
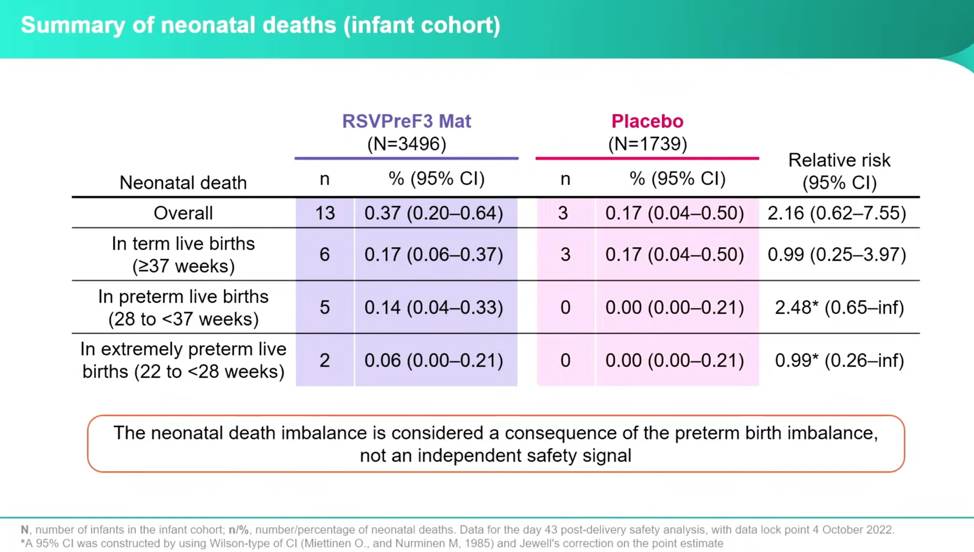
Even though Dieussaert downplays this data in her presentation, asserting it is part of the primary safety signal of preterm birth, the investigators found twice as many babies died in the vaccine group as in the placebo group. Dieussaert reassures that the number of at-term deaths occurred at the same rate between the groups, which meant outliers were all due to preterm births and should not be considered a separate safety signal. Her discussion, then, dismisses the deaths and focuses on preterm births. But the numbers seem to tell a different tale: the total deaths of vaccinated to placebo is 2.16 relative risk, versus the preterm births at 1.38.
Preterm Births Are the Main Signal
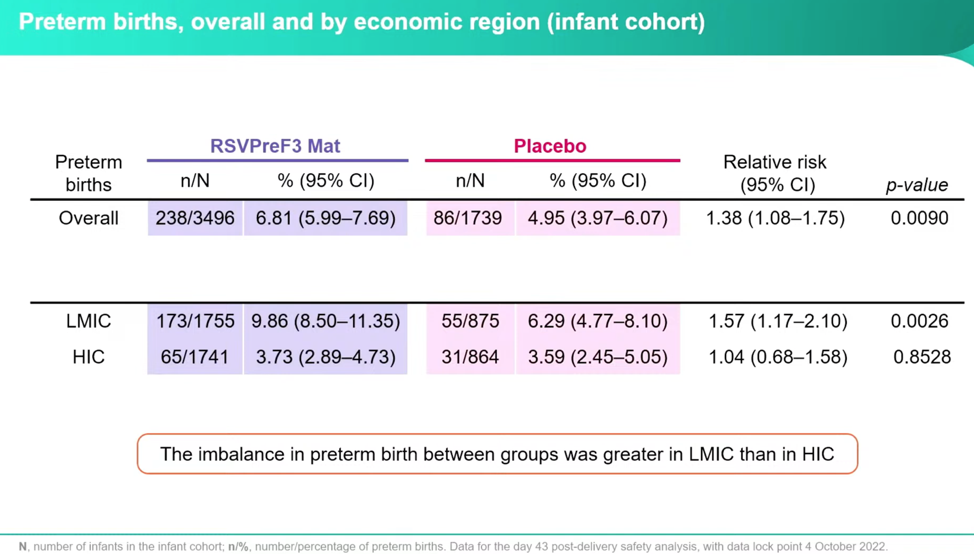
To look at the signal further, they evaluated the breakdown of preterm births by economic status and found that the greatest risk was in Low-Middle Income Countries (LMIC)—this is important, and I’ll get the why in a minute:
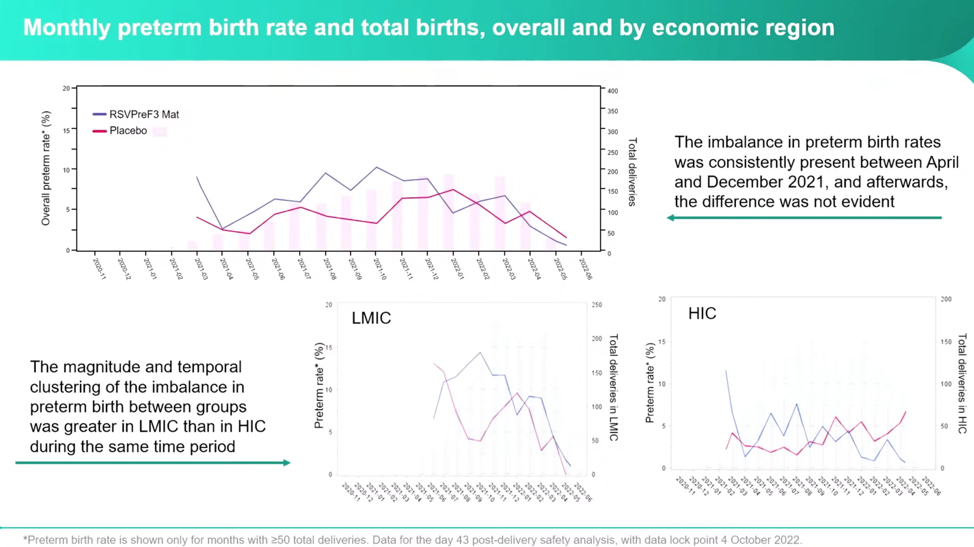
The investigators noticed the largest differences were between April 2021 and December 2021, and then became relatively equal between the vaccine and placebo groups.
Signal Intensified During Covid Vaccination Push
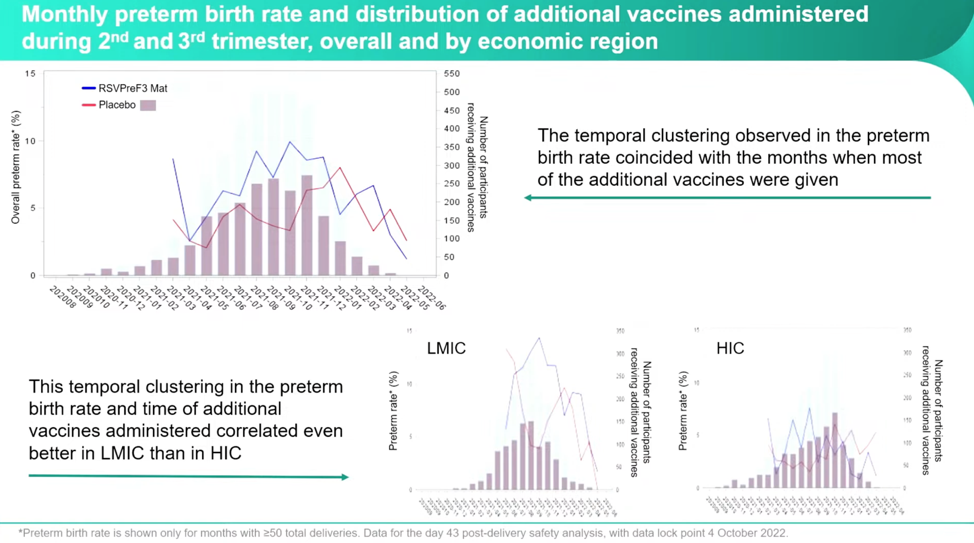
According to Dieussaert, the investigators questioned: “What happened in these months that could have contributed or could explain the imbalance that we’ve seen? And then you immediately think about the Covid pandemic, Covid vaccinations that were recommended during that time.” She added that they had enrolled participants year-round during the pandemic, which is why they looked at additional vaccines. Dieussaert included a slide full of other avenues they had pursued cause without finding any other correlations. During the trial, participants could receive other vaccines “two weeks before the investigational vaccine or two weeks after.” The investigators found a variety of types and timelines for the administration of additional vaccines. They plotted the RSVpreF3 vaccine and the placebo groups with the numbers of additional vaccines received against the number of preterm births, and a clear pattern emerged:
If you look at the RSVPreF3 group (blue line) against the number of participants receiving additional vaccines, you can see the clusters forming, especially in the LMIC.
Low-Middle Income Countries Have Higher Risk
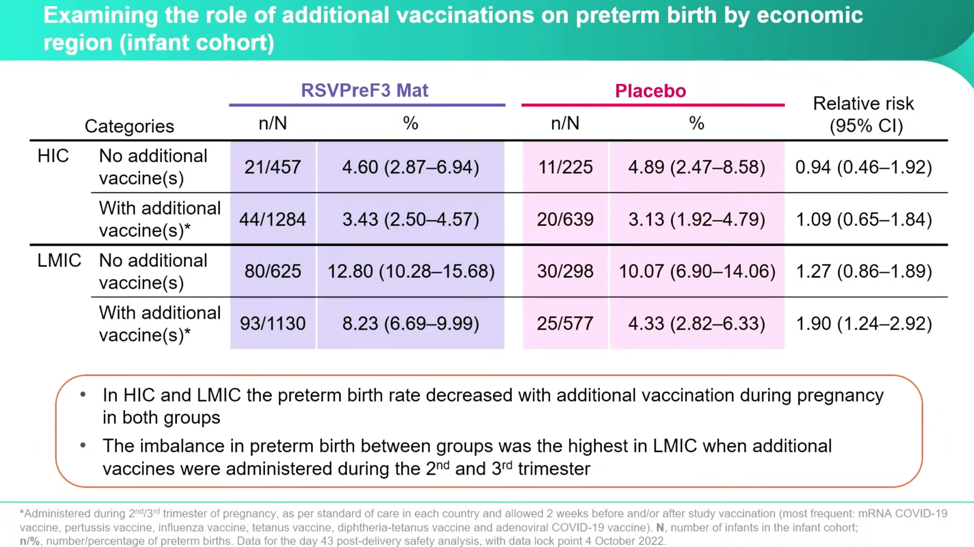
Despite some very concerning numbers, Dieussaert reassures conferencegoers that even though the imbalance increased between the RSVPreF3 vaccine group and placebo group, the “administration of additional vaccines during pregnancy decreased the preterm birth rate in both groups.” However, this is only half the truth if you don’t parse this out by country income status. Once separated out into HIC and LMIC, it’s clear that the real horror is happening in the LMIC and that it’s happening at a higher relative risk both with the RSVPreF3 and with additional vaccines at a startling rate:
Dieussaert explains, “The highest relative risk that we have observed here is in low-middle income countries when women received additional vaccines, we have a relative risk there of 1.9”—this is double the relative risk in HIC with no additional vaccines.
Data Shows Cumulative Vaccines Increase Risk of Preterm Birth
Considering that RSV trials are one of the first to be designed specifically for pregnant women, these data are extremely important. What they did right: using a saline placebo, halting trials at a danger signal, and analyzing potential environmental relationships for the increased preterm births. However, as the investigation is still underway, it would help future and current vaccines to have more data on the causes of death, rather than grouping them as all preterm, to unfold the mechanisms causing preterm births and extend adverse event follow-ups beyond the study design of six months post birth for the mothers and 12 months for the infant. Of course, we need to know why there is such a disparity of preterm births between LMIC and HIC and if the deaths in the trial also correlated with countries of high or low-income status.
Dieussaert closes her presentation with a lingering assertion that these data could help to evaluate overall benefit-risk ratios for future maternal vaccines. What this evaluation of a failed trial does, is not only looking at how an mRNA-based platform vaccine affects the outcomes of pregnancy but also the potential risks of cumulative vaccines during pregnancy. This is an important concession—that we need to evaluate the safety of vaccines and their cumulative effects on pregnant women and beyond. A traumatizing thought remains: Pfizer’s maternal RSVPreF vaccine, which finishes its phase 3 trial in November 2023, uses the same technology but seemingly has no safety signals. Somehow, it’s doubtful that they are just that good.
*All slide images are from:
RSV ReSViNET Foundation. (2023, February 22). Resvinet Conference 2023 Day 2 [Video]. https://www.youtube.com/watch?v=P45AbqFeYBY&t=28060s
Dieussaert image from: https://www.linkedin.com/in/ilse-dieussaert-ba223ab0/